Antigen-Specific T Cells to Human Cytomegalovirus (CMV)
Our scientists were among the first to develop custom antigen-specific T cells for research use and have created them against a diverse range of targets, including viral, tumor, and self-antigens.
We’re now developing the next generation of antigen-specific T cells to target some of today’s most prevalent and challenging diseases. More on that below. But first, a primer on antigen-specific T cells.
The Role of Antigen-Specific T Cells in Disease Research and Treatment
T cells are a major player in the immune response in various diseases, including cancer and pathogenic infections, and are mediators in inflammation and autoimmunity. A large body of research has been conducted over the past few decades to manipulate T cell responses, leading to many clinical strategies including cancer vaccines, T cell adoptive therapy, CAR-T therapy, immune-oncology, and the use of regulatory T cells for autoimmune diseases.
Antigen-specific T cells are an important tool in T cell biology research and potency assays. Investigators can isolate them directly from the disease site (e.g., tumor-infiltrating lymphocytes (TIL) or inflammatory lesion infiltrate populations), but these procedures often yield a limited number of cells due to low cell frequency and lack of robust isolation methods.
Alternatively, antigen-specific T cells can be generated in vitro using antigen-presenting cells pulsed with whole antigens or peptides. However, this process is often time-consuming with a low success rate.
About Ignyte Bio’s Antigen-Specific T Cells
Ignyte Bio offers antigen-specific T cells for research use you can rely on.
- Typically generated using multiple in vitro stimulations with peptide antigens
- Not immortalized or genetically modified, so they more closely mimic physiological T cells
- Specificity analyzed using the cognate peptide/MHC tetramer binding as well as interferon-gamma (IFN-γ) secretion and cytotoxicity assays.
Announcing Ignyte Bio’s Newest Antigen-Specific T Cells : anti-CMV CD8+ T Cells (HLA-A*02 and HLA-B*07 Restricted)
Human cytomegalovirus (HCMV) is a ubiquitous beta herpesvirus that infects >50% of the world’s population. After primary HCMV infection, the virus persists lifelong in a latent state in cells of the myeloid lineage and under the control of the immune system. HCMV reactivation can, however, cause serious disease in immunocompromised individuals, such as patients with advanced human immunodeficiency virus (HIV) infection and patients who have undergone bone marrow transplantations. Evidence from animal models and from studies of immunosuppressed humans indicates that virus-specific T cells have a prominent role in protection against CMV disease.
HCMV seropositive healthy donors typically have high frequencies of human leukocyte antigen (HLA)-restricted HCMV-specific memory T cell precursors in peripheral blood. CD8+ T cells (also known as Cytolytic T Lymphocytes [CTLs]) that target a matrix phosphoprotein of 65 kDa (pp65) (Wills et. al., 1996) were identified as the major responder T cells against HCMV. The high abundance of HCMV-specific T cells makes peripheral blood mononuclear cells (PBMC) from seropositive donor a suitable cell population in an in vitro recall assay (see our blog on Recall Antigen Response As A Model of In Vitro T Cell Response). T cells specific for several HCMV epitopes have been identified and characterized.
The first HCMV-specific T cell epitope identified was the pp65 (495-503): NLVPMVATV, restricted by HLA-A2, or HLA-A*02) (Wills et. al., 1996), the most frequent HLA-A family present in most ethnic groups (Ellis et. al., 2000). CTLs that recognize different pp65 epitopes have subsequently been identified, including pp65 (417-426): TPRVTGGGAM. This particular CTL response is restricted by HLA-B7, or HLA-B*07.
In the past few years, Ignyte Bio has offered HLA-A*02-restricted Antigen-Specific T Cells (ASpecT) to HCMV pp65 (495-503): NLVPMVATV from a couple of donors. We can now offer the HLA-B*07-restricted ASpecT to HCMV pp65 (417-426): TPRVTGGGAM.
Figure 1 shows anti-CMV pp65 (495-503, NLVPMVATV, HLA-A*02 Restricted) T cell-mediated specific IFN-γ secretion and specific lysis when HLA-A-02:01+ target cells were incubated with the CMV pp65 (495-503, NLVPMVATV) peptide but not the control HPV E711-20 (YMLDLQPETT) peptide. Figure 2 shows anti-CMV pp65 (417-426, TPRVTGGGAM, HLA-B*07 Restricted) T cell-mediated specific IFN-γ secretion and specific lysis when HLA-b-07:02+ target cells were incubated with the CMV pp65 (417-426, TPRVTGGGAM) peptide but not the control CMV pp65 (495-503, NLVPMVATV) peptide.
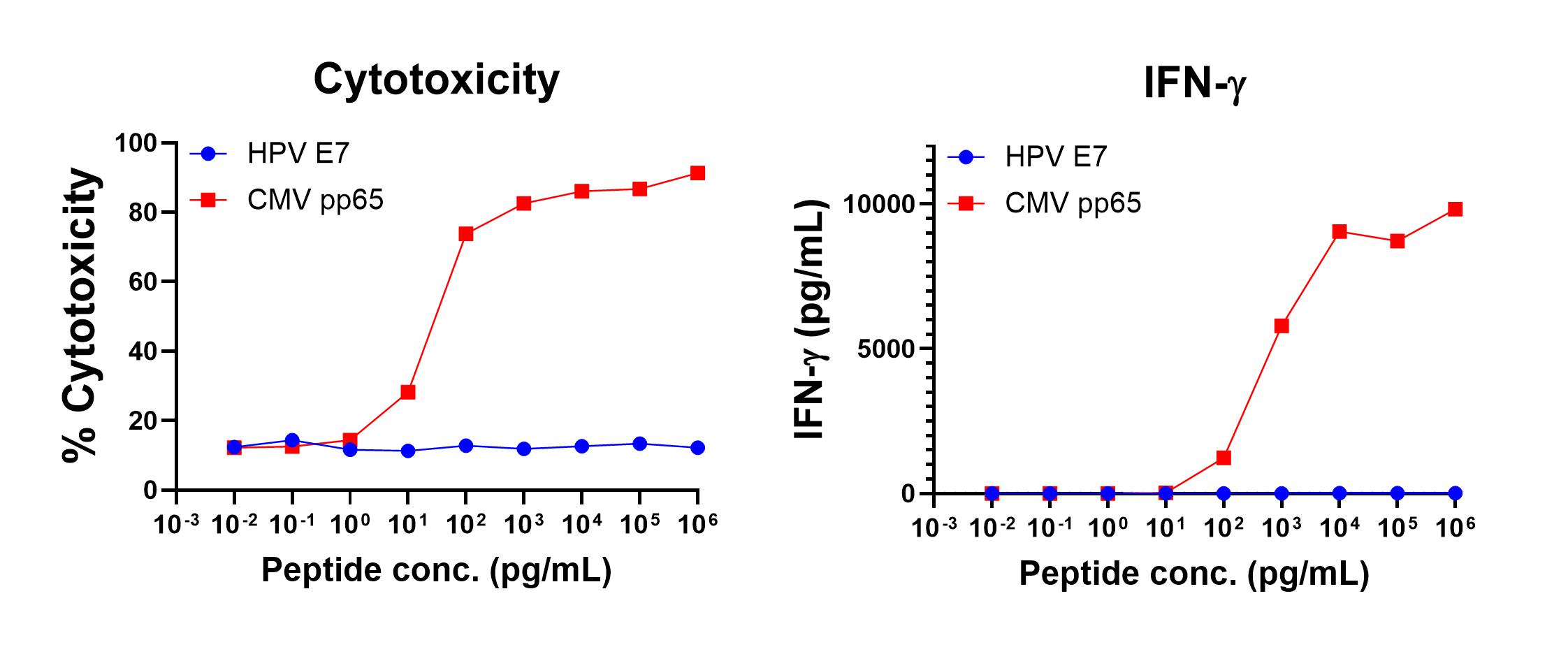
Figure 1. IFN-gamma Secretion and Cytotoxicity of HLA-A*02 Restricted anti-CMV pp65 (495-503, NLVPMVATV). Freshly thawed anti-CMV pp65 (495-503, NLVPMVATV) T cells were plated at 2 x 104 cells/well in the presence of 2 x 104 T2 cells, an HLA-A-02:01+ B-LCL (ATCC, Manassas, VA) and different concentrations of CMV pp65495-503 (NLVPMVATV : CMVpp65 peptide or HPV E7 11-20 (YMLDLQPETT): HPV E7 control peptide in a 96-well round bottom plate. After 18-24 hours of incubation a t 37oC, 5% CO2, culture supernatants were harvested and assayed for IFN-g using the Lumit™ IFN-γ Immunoassay (Promega, Madison, WI). IFN-γ concentration is plotted against CMV and control peptide concentrations (right graph). Remaining cells in each well were stained with the viability dye 7-AAD and analyzed using flow cytometry. % Cytotoxicity (= % 7-AAD+ target cells) is plotted against the respective peptide concentrations (left graph).
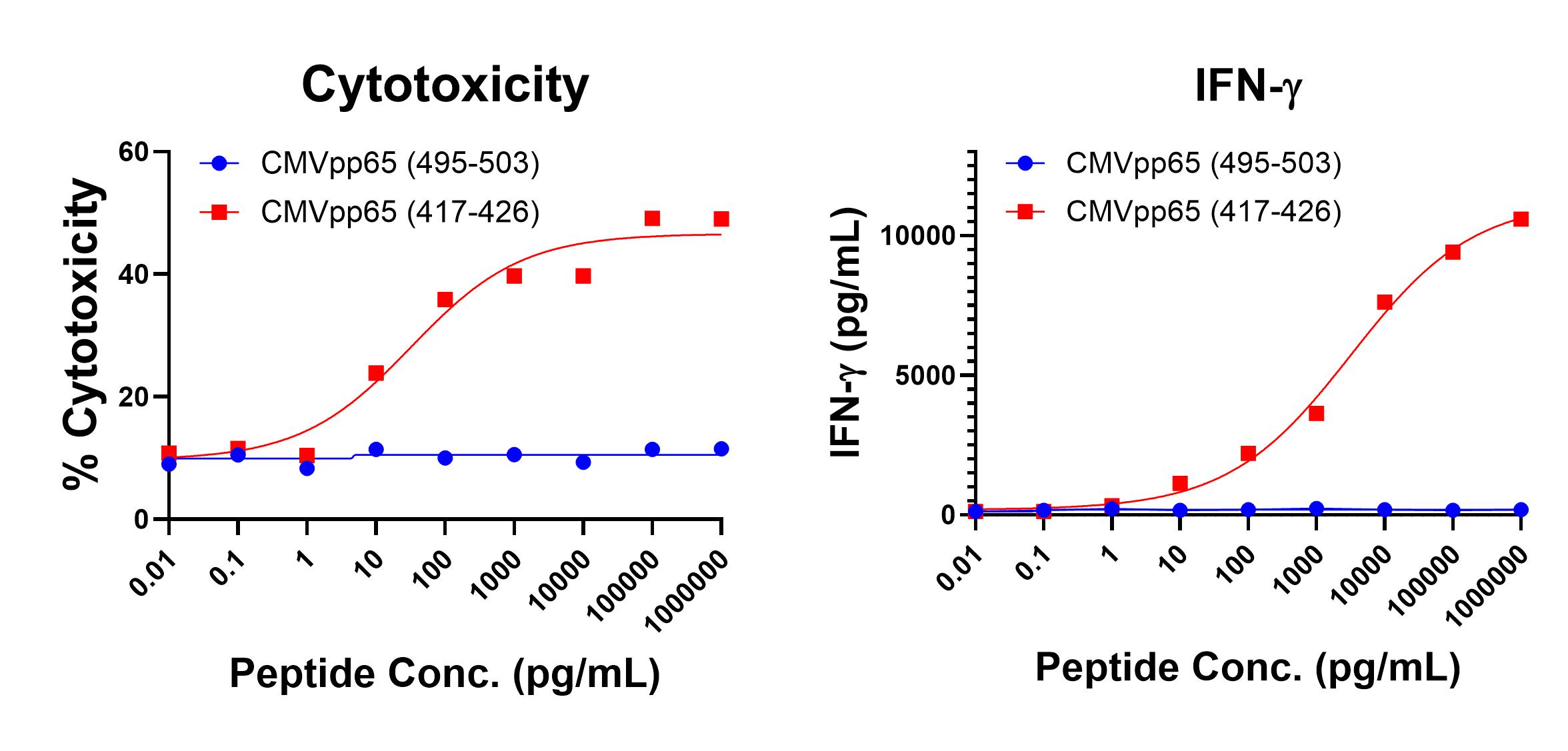
Figure 2. IFN-gamma Secretion and Cytotoxicity Data of HLA-B*07 Restricted anti-CMV (417-426, TPRVTGGGAM). Freshly thawed anti-CMV (417-426) T cells were plated at 2 x 104 cells/well in the presence of 2 x 104 HLA-B+ 0702+ allogeneic B-LCL (Ignyte Bio, Seattle, WA) and different concentrations of CMV417-426(TPRVTGGGAM): CMV (417-426) peptide or CMV 495-503 (NLVPMVATV): CMV (495-503) control peptide in a 96-well round bottom plate. After 18-24 hours of incubation a t 37oC, 5% CO2, culture supernatants were harvested and assayed for IFN-g using the Lumit™ IFN-γ Immunoassay (Promega, Madison, WI). IFN-γ concentration is plotted against CEA and control peptide concentrations (right graph). Remaining cells in each well were stained with the viability dye 7-AAD and analyzed using flow cytometry. % Cytotoxicity (= % 7-AAD+ target cells) is plotted against the respective peptide concentrations (left graph).
